Illustrated by @scienceblocks, using the following images -
car by OpenClipart-Vectors and gangsterby mohamed_hassan.
CC0
Previously on wound healing
In my previous post, on dynamics of wound healing I told you about different phases healing and cellular players that are required during process. I described the immediate events that happens during healing. The first to come is hemostasis which stops excessive blood loss by vasoconstriction and by recruitment of platelets which forms the fibrin clot. This is followed by vasodilation and recruitment of immune cells. In the meantime, the epithelial cells in epidermis start to proliferate and migrate to close the would. Working hand in hand with epithelial cells are activated fibroblasts in the dermis, which degrades fibrin, and deposit the lost extracellular collagen matrix. They then hold tightly to fibers of newly deposited matrix and contract the wound accelerating the healing process.However, sometimes during the healing process the fibroblasts fail to deactivate and die. This may happen due to chronic wound signals or repetitive injury or insult to the organ. This persistent activation of fibroblast leads to formation of hypertrophic scars and even fibrosis - which is hardening of the tissue causing loss of organ function. I described in my previous post, how fibrosis is either cause or consequence of many chronic diseases and how it is one of the major cause of death globally. I also mentioned that solid tumors are fibrotic tissues. Probably because they exploit the tissue repair and development machinery; and this is what we are going to look at closely in this post.Cancer - the rebellion of cells
You think becoming a cancer cell is easy? You think the body of the organism will let you win so easy? I guess not. If you were a cancer cell, you are like this rebel - probably the one who wants to seize the means of production and then take over the world. But, you have this entire world against you. And, no you can't take the world by surprise, because they have always anticipated a rise of such rebellion. They have defences against you, built during millions of years of evolution, ready to be deployed.You thought, a single mutation will help you multiply uncontrollably. Well no you have a hell lot of intracellular checkpoints in place. You mutate one of the oncogene, you have a tumour suppressor gene in place. You mutate a tumour suppressor gene to escape cell death, you have redundant mechanisms to take care of it. It is now well recognised that you need multiple genetic insults to create the first tumour initiating cell (Chial, 2008). In fact, as we talk, there will be at least 1 new mutation that gets stabilised in one of your cells. By the end of the day there will be a lot of cells in your body with at least one new mistake (Risques and Kennedy, 2018). If you look closely in your gut you will find that upto 37% and 80% cell accumulate TP53 and Notch1 mutation respectively (Martincorena et al., 2018), and yet it's not necessary that you get a tumour, let alone cancer.Even, if some set of mutations and epigenetic changes causes the tumour, you still need much more than just dividing like crazy to cause cancer. The soldiers of body, aka immune cells are going to treat you like a felon and kill you. So you need to fool them or corrupt them in your favour. You need to become more notice, travel through the body. You need to spread your business to multiple tissues to become cancer. So basically you need to have the environment of body in your favour. From a local goon, you need to become an inter-tissue mafia Don, with network all over the body.What kind of environment exists in body where cells are allowed to multiply and immune system aids their growth and migration? Well, one such environment wound healing process. If a tumour cell can make use of such a process in an exaggerated manner, it's in business.Exploitation of wound healing machinery
A solid tumor is an overhealing wound, is not a new idea. As early as 1863, Rudolf Virchow, proposed that chronic irritation and previous injuries sites in the tissue, favours tumorogenesis (Schäfer and Werner, 2008). In fact, it is known that both persistent inflammation of the tissue and injury sites are hot spots for fibrosis and tumors. In 1972, Alexander Haddow, molecular drew parallels between wound healing and carcinogenesis. Then in 1986, in publication Tumors: Wounds that do not heal, Harold F. Dvorak showed how anatomy of tumor resembles that of a fibrotic tissue or an overscarring wound. That is to say that any solid tumor is an epithelial tissue, surrounded by fibrotic connective tissue called - tumor stroma.So the basic difference between a perfectly repaired wound, a scar, a fibrotic organ and tumor, lies in extent of exploitation of wound healing machinery. In a regenerating organ, you heal via bringing the tissue back to their initial state. In an imperfect repair as we usually witness on our skin, healing ends in remodeling phase, leaving a tiny bit a hypertrophic scar behind. However, when then fibroblast cells that remodel the matrix of any tissue during healing, remain persistently activated, you get fibrosis. And when the fibrosis is caused by rebellion epithelial cells, that wants to divide and conquer the body, you get a tumor.Nevertheless, that's the bigger picture. But the main question remains that how is the machinery of wound healing being exploited by the cancer cells. To understand that we can revisit the wound healing phases once again and see how the different phases of normal healing process are exaggerated in cancer.Phases of wound healing and their exploitation by cancer
.png)
Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008.| Public Domain.
The fibrin clot and tumor
.jpeg)
Image by sportEX journals | CC BY-ND 2.0.
Making friends with immune cells
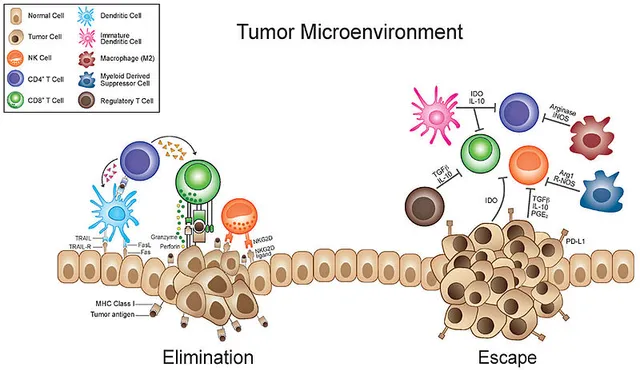
Image by Fronteirs in Oncology | CC BY 3.0
The proliferative phase, re-epithelialization and metastasis
During the normal healing process the stem cell niches are activated. The epithelial cell numbers expand via rapid proliferation. So, does in cancer. Just that cancer cells don't have an off switch. The cancer initiating cell gives rise to rapidly proliferating proginitor cells. The cancer initititing cells maintains it's niche, just like stem cells would (Arnold et al., 2014). These cancer stem cells also initiate inflammation, which act as a feedback loop to hijack the wound healing pathway.The downregulation of caspase8, is an important event in initiation of wound healing response. In 2009, Jamora lab, demonstrated that knocking out caspase8 is enough to recapitulate all the phases of wound healing in entire skin of the mouse. A similar downregulation of caspase8 in epithelial cells of carcinoma, is also hallmark of many tumors.Another common signature of wound healing and cancer is activation of transcription factor Snail/Slug. In our lab we find overexpressing snail in epidermis of mice recapitulates wound healing, causes dermal fibrosis, and make mice highly likely to get squamous cell carcinoma (Du et al., 2010). Snail/Slug also increase survival of cancer stem cells. It also makes them more fibroblast like and hence more motile. Hence, Snail aids in metastasis.Moreover, re-epithelialization of wound requires a two state switch. One that turns on migration of epithelial cells and one that turns it off. Sundaram, 2017, showed that the migration is initiated by activation of FSTL1. The FSTL1 off switch is dependent of its mRNA degradation by microRNA, mir-198, during normal healing. However, epithelial cells of carcinoma, the mir-198 switch is missing. This helps the cancer cells to migrate uncontrabally and successfully breaching the barrier of their primary site.Tumor stroma - hijacking the construction workers
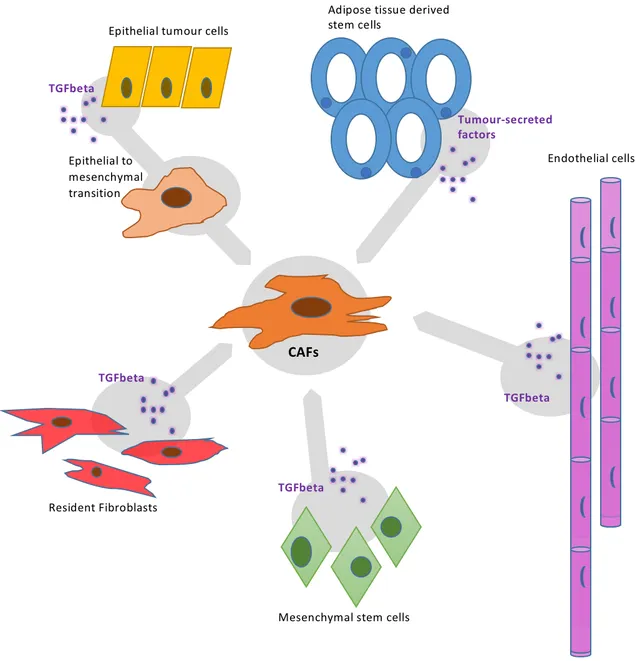
Image by Alice Ayers | CC BY-SA 4.0.
Summary
In a nutshell, what we saw in this discussion is that cancer hijacks wound healing machinery. It fools a lot of cells into trying to repair a wound that would never heal. A wound environment is quite favourable for growth and metastasis of cancer. Understanding tge simalarities and differences between a wound and tumor, provides an oppurtunity to make more efficient treatment strategies. Especially, strategies that targets tumor stroma.As I describe my research further, we will bump back into this topic again. I think for today, this much information should suffice.About steemstem
But before I go I would like to mention about the steemstem platform. Well, if you love reading and writing interesting science articles @steemstem is a community on steem that support authors and content creators in STEM field. If you wish to support steemstem do see the links below.You can vote for steemstem witness here -Quick link for voting for the SteemSTEM Witness(@stem.witness)Delegation links for @steemstemQuick delegation links: 50SP | 100SP | 500SP | 1000SP | 5000SP | 10000SP).Delegating to @steemstem gives ROI of 65% of the curation rewards.References
- The biology and physics of wound healing (part 1 - the cellular players and dynamics of healing)
- Fibrosis - an overscarring wound
- Chial, 2008. Tumor suppressor genes act as “brakes” to stop cells before they can travel down the road to cancer. A loss of function mutation in these genes can be disastrous.
- Risques RA, Kennedy SR (2018) Aging and the rise of somatic cancer-associated mutations in normal tissues. PLoS Genet 14(1): e1007108. doi:10.1371/journal.pgen.1007108
- Martincorena et al., 2018. Somatic mutant clones colonize the human esophagus with age
- Matthias Schäfer & Sabine Werner, 2008. Cancer as an overhealing wound: an old hypothesis revisited
- Alexander Haddow, 1973. Molecular Repair, Wound Healing, And Carcinogenesis: Tumor Production A Possible Overhealing?
- Harold F. Dvorak, 1986. Tumors: Wounds That Do Not Heal
- Obonai et al., 2016. Tumour imaging by the detection of fibrin clots in tumour stroma using an anti-fibrin Fab fragment
- Gambhir S, Vyas D, Hollis M, Aekka A, Vyas A. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies.
- Base calling neutrophils to destroy tumors!
- Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273(1):329-43.
- Muluaditan et al., 2018. Macrophages are exploited from an innate wound healing response to facilitate cancer metastasis
- Regulatory T cells in cancer. Marc Beyer and Joachim L. Schultze, 2006.
- Givel AM, Kieffer Y, Scholer-Dahirel A, et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun. 2018;9(1):1056. Published 2018 Mar 13. doi:10.1038/s41467-018-03348-z
- Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis. 2015;8:1-13. Published 2015 Jan 29. doi:10.4137/CGM.S11286
- Lee et al., 2009. Dynamic expression of epidermal caspase 8 simulates a wound healing response
- Du F, Nakamura Y, Tan TL, et al. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70(24):10080-9.
- Sundaram et al., 2017. EGF hijacks miR-198/FSTL1 wound-healing switch and steers a two-pronged pathway toward metastasis
- Sinha et al., 2018. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue
- Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel). 2015;7(4):2443-58. Published 2015 Dec 11. doi:10.3390/cancers7040902
