The family of acids is very broad, we are aware of them because, unlike the bases, many of these compounds are identified generically or commercially with the word "acid", for example battery acid, ascorbic acid (vitamin C), acetic acid (vinegar), acetylsalicylic acid (aspirin), to name a few.

Example of acid. Image edited by the author, image of citus fruits of public domain.
Acids are chemical compounds whose main characteristic is its very large capacity to yield protons or to accept a pair of electrons. This characteristic is derived from the most accepted definitions of these compounds, one of the first was established by Arrhenius, which defined them as "a compound that contains hydrogen and reacts with water to form hydronium ions"[1], the problem of this definition is that it links them to a single solvent, water. An important contribution to the understanding of what an acid represents was independently introduced by Thomas Lowry and JohhannesBrönsted, considering boththat the property responsible for the characteristics of acids was the transfer of a proton (a hydrogen ion) from a substance to another, leaving Brönsted-Lowry's definition as "an acid is a proton donor"[1].
These definitions allow us to have a global idea of the chemical characteristics of this type of compounds, but there are many substances that have no hydrogen in their composition yetbehave as acids when neutralizing substances with basic characteristics, so they stop fulfilling the condition of the transfer of a proton, therefore, another concept was proposed by Lewis to describe them, according to his definition an acid is "a chemicalkind that can accept one pair of electrons from another"[2]. StillBrönsted-Lowry’s definition is the most widespread.
In a natural way we can recognize acids for their bitter taste, in nature we find them in many fruits, especially those we know as citrus. For example, citric acid gives its characteristic flavor to the juice of lemon or strawberry. And as it happens with these fruits, the greater the degree of acidity they contain, the lower our capacity to tolerate the taste of them. Therefore, the sense of taste provides us with a sensory way to detect the presence of acids in food.
But acids are not only found naturally in fruits, we will also have heard about fatty acids, which are organic acids of long carbon chains, present in oils and fats. And surely many have experienced the sensation described as heartburn, that burning sensation in the throat. Well, the gastric juice contained in our stomach, is a secretion of several specialized cells and contains in its composition hydrochloric acid, in addition to water, various salts and enzymes[3]; and the burning sensation is caused by the reflux of food into the esophagus.
Beyond fruits and food, acids have important uses, at home hydrochloric acid is part of many cleaning products and perhaps sulfuric acid is the acid with greater industrial use, is used in the manufacture of agrochemicals, to acidify oil wells, in the metallurgical industry is used for the cleaning of metals, not to mention that it is the electrolyte used in car batteries.

Batetery for vehicles. Source: maxpixel.net, Licence CC0.
And what are the characteristics of acids?
According to the existing definitions, acids have the following characteristics:
- They are capable of generating H+
Hydrogen chloride (HCl), acetic acid (CH3COOH) and methane (CH4) contain hydrogen atoms in their structure, but only the first two can transfer them to other substances, and in solution they release hydronium ions while methane does not do it, although it contains hydrogen, it cannot be easily transferred.

A convenient way to recognize an acid is by its chemical formula, usually the hydrogen atom is written that can be given as the first element of the formula (HCl, HNO3, H2SO4), except in organic acids, such as acetic acid, in which is written the formula to better represent the carboxyl group (-COOH). In this way we can see that methane or ammonia (NH3) for example, correspond to acid formulas.
- They have hydrogens with poor electronic density
In the case of methane, none of its hydrogen atoms has electronic deficiency, because the difference in electronegativity between the carbon atom and hydrogen is not very large, but if we compare it with a molecule of hydrogen fluoride (HF), the difference in electronegativity between the hydrogen and fluorine atoms is so great that electrons spend more time in the electronic atmosphere of one atom than in the other, this unequal distribution of electrons results in a shift of the electron density of hydrogen to fluorine.
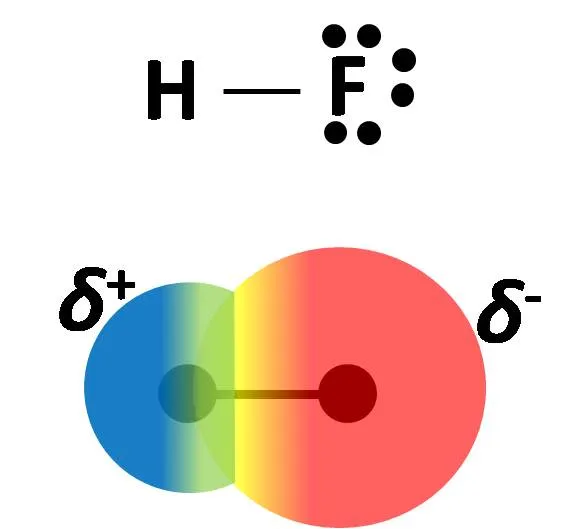
The image shows the Lewis structure for hydrofluoric acid and the electron density mao for the molecule, the red color region is the area with the highest electron density. Own image.
- Strength and constant acidity
Acids, as well as electrolytes, can be classified as strong or weak, this is related to their ability to yield a proton. In general, it is known as a strong acid all those that can dissociate in their entirety while the weak acids are all those that are partially dissociated[4]. And this capacity is measured in terms of the equilibrium constant, in the case of acids, better known as the acidity constant (Ka), which is the equilibrium constant of its reaction with water. Strong acids, such as hydrochloric acid, easily transfer protons, so in equilibrium will be displaced towards products, its equilibrium constants are so great that they are considered infinite, that is, that their reaction is irreversible. While in weak acids, protons give so easily that their equilibrium constant is less than one, for example the Ka of acetic acid is 1.8 * 10-5, and others have much smaller values , such as hydrocyanic acid (Ka = 4.9 * 10-10)[1]; that is, the balance of its reaction with the water is displaced towards the reactants. Incomplete deprotonation in weak acids explains why they have a higher pH than a strong acid at the same concentration. Therefore, it can be said that the greater Ka, stronger the acid.
- Generate conjugated pairs
Once the protons of an acid are ceded, the acid is transformed into what is known as a conjugate base; that is, a chemical kind that now has the capacity to accept protons or yield a pair of electrons. If we observe the reaction of acetic acid in water:
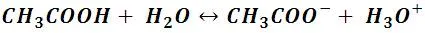
It can be deduced that the resulting CH3COO- ion now has the capacity to accept H + ions, which makes it a base; and in fact, it is such a good proton acceptor that it is cataloged as a strong base. In general, weak acids generate strong bases and vice versa, strong acids generate weak bases, which is defined by conjugate acid-base pair.
- They can have positive charges
As I mentioned earlier, not all acids have hydrogen in their chemical composition, but they can also have another type of atom with the ability to accept electrons. Consider metal cations such as Na+ or Cu2+, these can be considered acids, since in solution they can accept coordination bonds fromrich kindelectron. For example, in the reaction of Na+ ions with OH- ions to form sodium hydroxide:

OH- ions have the ability to accept protons, which are bases, and as itcan be seen, the oxygen atom of this molecule has pairs of free electrons, complying with the definition of Lewis base, in this way, the ions Na+ constitute the acid in the reaction. This behavior can be extrapolated to the hydration of the Cu2+ ion, when Cu2SO4 is dissociated in water, each Cu2+ ion is associated with six water molecules to form the aqueous Cu[H2O]2+ complex, so that the Cu2+ ion acts as the kindthat accepts a pair of electrons, the acid in this case.
So, all kinds that experience this behavior are known as Lewis acids, while those that donate protons are known as Brönsted acids.
- Your solutions have pH values less than 7
From what we know then, an acid solution contains a large amount of hydronium ions, so the acidity of the solution depends on the concentration of this ion in the solution. The pH scale is the most practical criterion that chemists have recognized to establish the acid or base condition of a solution; pH is defined as the negative logarithm of the molarity of the hydronium ion:
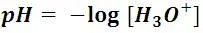
The negative sign means that the larger the concentration of the hydronium ion, the lower the pH. Specifically speaking, any solution whose pH is less than 7 (which is the pH of pure water) is considered as acid.
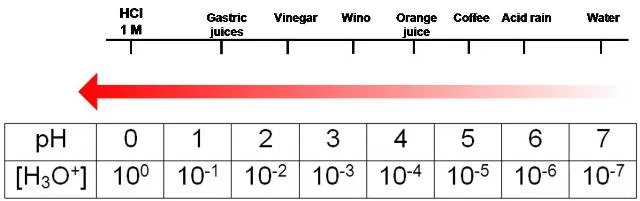
pH scale for the acid region representing the pH of some of common substances. Source: adapted from Chemistry Libre Texts, library with content under license CC BY-NC-SA 3.0.
- Neutralize bases
Acids have the property of reacting with the bases to form a salt as a product, therefore these reactions are known as neutralization, because they mutually neutralize their acidic and basic properties.

Although in general the reaction products can be harmless, as in the neutralization of hydrochloric acid with sodium hydroxide, which produces the sodium chloride salt (common salt), in other cases we must be careful, for example; In our homes it is common to have cleaners that are solutions of hydrochloric acid (HCl) and some that are solutions of ammonium hydroxide, the vapors of these compounds have the characteristic of reacting producing a cloud of ammonium chloride, according to the following reaction:
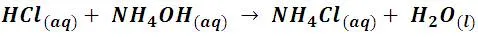
Ammonium chloride forms a toxic cloud that can cause irritation of the respiratory tract.
Final considerations
Acids have great industrial applications and multiple applications in our daily lives. Its application depends on its characteristics, but we must always have the proper precautions when using them, either as concentrated acids or as their solutions, which are used in household cleaners.
When it is indicated that the acids have a bitter taste, it is always that their concentration is low enough so as not to irremediably damage the tissues of the tongue, because we must remember that they are also quite corrosive.
At this time we have all heard about acid rain, and its effects. This is produced by the reaction of non-metallic oxides such as SO2, NO in addition to CO2 with water, forming sulfuric acid, nitric acid and carbonic acid; what gives the rainwater a pH close to 6, which is able to cause severe damage to forests and deteriorate statues and ornaments of buildings that are made of marble or limestone.

Damage caused to a forest by acid rain. Source: Lovecz, public domain image.
References
1. Atkins, P., Jones, L. (2006). Principles of chemistry.
2.Chang, R. (2002). Chemistry.
3. Wikipedia.com. Jugo gástrico.
4. Wikipedia.com. Acid.
