Well, It has been quite some time, since I had time on my hands to write content. The pandemic changed a lot of things for me, including the amount of time I had in my hands. Ranging from volunteer work to assist in setting up a Covid19 testing facility, to shifting my research gears to antiviral drug development It has. It has been a roller coaster in short. I could have started writing today again discussing some science, but that would be abrupt. Also, I am sure there are many new faces on the platform that I have not interacted with. Hence, I thought why not share my experience with you. How it was working in a Covid19 testing facility during the very early days. How is it inside the walls of BSL3 where we work with the virus?

When it all started

We had been hearing about Covid-19 cases since January 2020, when it all began in China. However, the cases have not yet come to India (or at least we were not aware of any). Then the reports of cases started coming up in India 1. Soon thermal scanning started on all airports. Our campus started preparing for what now seemed to be inevitable. Weeks before the national lockdown all measures - from masks to social distancing, to dividing each space into sectors (to minimize the interaction between individuals) were put in place. The national lockdown was announced by the prime minister on 25th March 2020 2
Diving in

For the next 15 days, we did not go to the lab. Well except for one volunteer to ensure everything in the lab (the reagents, the animals) was ok. But, they declared that Covid19 related research will be allowed on the campus. Also, the campus was about to start a Covid19 testing lab and we were told that we will be asked to volunteer.
Experiencing drug discovery and virology
It was in those 15 days that I decided that I will start an antiviral screening project in the lab. I did some reading. Maybe about 200-300 papers on virology, assays used and all the new research that was pouring in about Covid19. I and my colleague sent the project proposal to our mentor and got the green light.
The idea
High throughput screening
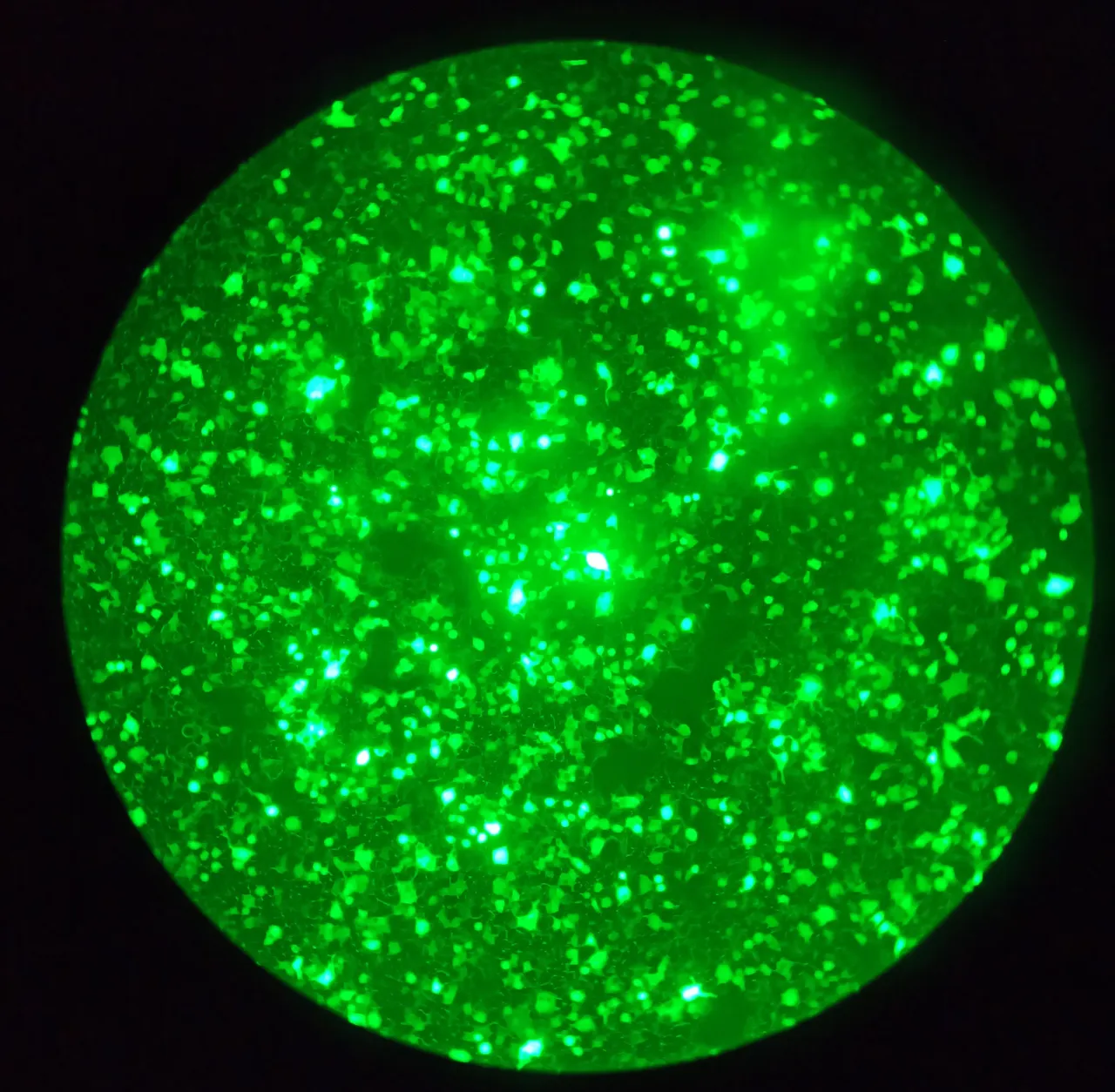
The idea was pretty simple. Design a replication-incompetent virus that expresses Sars-CoV-2's Spike protein on its surface ( a spike pseudovirus)3 but can't cause infection. The pseudovirus will carry a fluorescent gene. When the pseudovirus will infect a cell line that can be infected by the real virus as well, then the infected cells will shine green under the microscope. Well, if any molecule in there inhibits the viral entry or messes up the virus then the number of cells that shine green will be reduced. We will then measure the number of cells shining green in no treatment vs those being treated with 100s of compounds and extracts that we had in the library.
Now, this part was rather easy. We did not have to deal with the infectious SARS-CoV-2 virus at this stage. I did not have to wear that biosafety level 3 (BSL3) appropriate PPE and work in a negative pressure room. This turned out to be real smooth and fast. By end of 2020, we had 5 leads in our hands that could block the viral entry. But now came the challenging part. Will these leads work against the actual virus?
The real deal

The leads that can block the pseudovirus entry were now ready to be tested for their activity against the real virus. So all geared up I started this phase. Soon I will be testing what we have on the mice model susceptible to Sars-CoV-2 infection. For example, K18-hAce2 transgenic mice (which is a mouse that expresses a human version of Ace2 on its lungs cells)4.
In a nutshell, our idea is to generate a general antiviral drug not just against Sars-CoV-2 but against multiple respiratory viruses that pose threat to us in near future. Hopefully, we will find an antiviral that blocks the entry of the virus. The work is in progress. Not much can be revealed about it but fingers crossed, and hope it works out.
How was it like working in a testing lab
By mid of April 2020, we were asked to volunteer for setting up a Covid19 testing lab on the campus. We formed 5 teams to set up a testing facility.
- The scientific staff-The aliquoting team-The RNA extraction team-The RT-PCR team-The data management team
The scientific staff
Initially, I started in the scientific staff. Our job here was to carefully collect patient samples from the hospital and get them to the aliquoting team in the biosafety lab. It had to be done safely without contaminating ourself, or the environment and surfaces around us. I was trained for Biosafety level 3 protocols. Here we use to work in pairs of staff member type 1 and staff member type 2. Type 1 was responsible for handling the sample in fully covered PPE, while the member from staff 2 would assist to open all the doors and operate the lift so staff 1 member do not touch anything while handling the patient samples. The type 1 member would duff the PPE he used to carry the samples, and then donne the level 3 appropriate PPE. Then he would enter the BSL3 lab and hand over the sample to the aliquoting team.
The aliquoting team
The aliquoting team member would then open the vials with patient swabs inside the biosafety cabinet. He would read the patient ID and name on the sample tube and the scientific staff member would tally it with the list of patient IDs and names we received from the hospital and the central database. If the vial was leaked or if there was a mismatch between the name and ID between the vial and the database then the sample would be discarded. If everything seems to be fine then the aliquoting person would give a green light and a scientific staff member would update the status of all samples for the data team. Meanwhile, the aliquoting person would neutralize the virus, if any, and put the part of the liquid in the mother tube in a new vial which will be sent to the RNA extraction team.
The RNA extraction team.
The RNA extraction team as the name suggests was responsible for extracting all the RNA in the vial they received from the aliquoting team, using the RNA extraction kit provided to them. I know it sounds simple to put this in words, but their work was the most tedious and essential of all. The quality of extracted RNA mattered for everything that happened downstream. This extracted RNA was then sent to the RT-PCR team.
The RT-PCR team
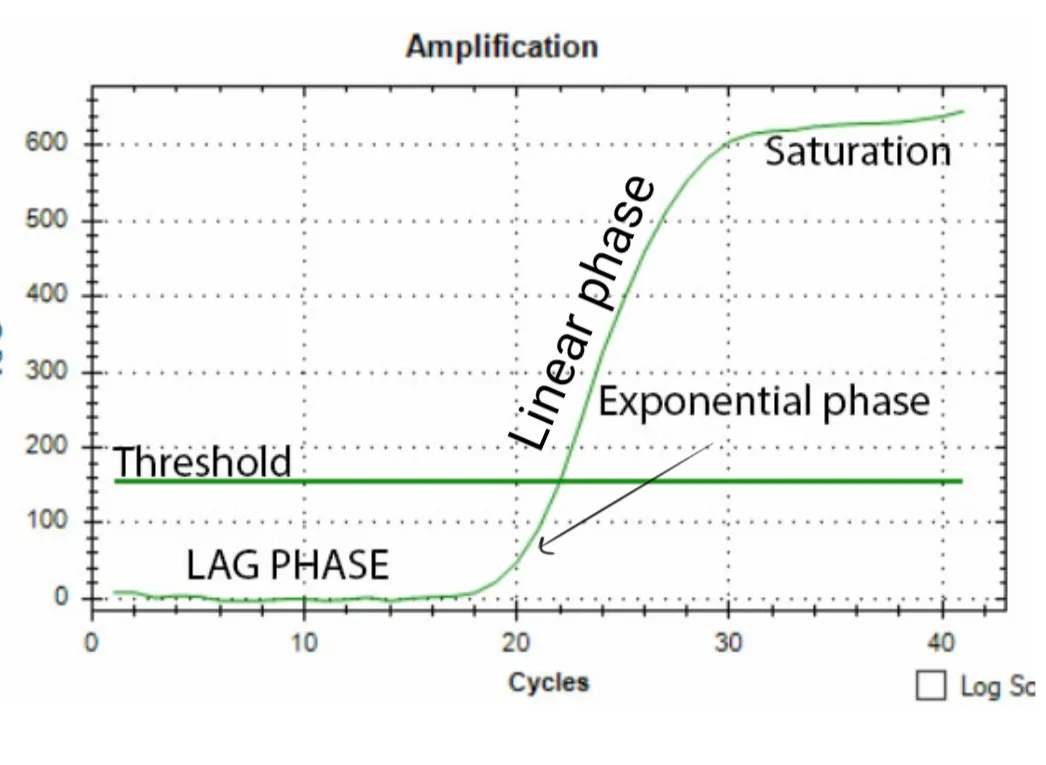
The RT-PCR team was responsible to carefully set the RT-PCR reaction according to the map of 96 well plates provided to them by the data management team. The map instructed which patient sample will go in which well. In the initial days, they would first run the RT-PCR for the human RNAseP gene and viral E gene. The human RNAseP gene was like an internal control. If everything from sample collection to RNA extraction went well, and the quality of RNA is good then this gene should be amplified and detected. If this gene was not detected (which means that ct value > 35) the data management team would classify this as an invalid result. If the ct value for RNAseP was less than 35 but that of the E gene was > 35 then the sample would be considered negative for covid19. However if E gene's ct < 35 it would be declared as a potential positive. The positive RNA would be further confirmed by another RT-PCR for viral orf1a and RdRp genes. If either or both of them showed amplification then the person would be declared covid19 positive 5.
This whole process took at least 2 days to give the result from the date of receiving the sample. That is provided we did not have a backlog. Well, at least that was the case before the multiplex RT-PCR kits came in6, 7. Since multiplex RT-PCR can detect multiple genes in a single run the process was now faster and results can be delivered in a day. Well almost! One limiting step still existed in data management, which was delaying the results.
Just in a matter of a week, we realized that we are not short of people in any other team but the data management team. Given my skills in data analysis, I opted to shift gears and moved to data analysis. Over there our tasks ranged from receiving the data from a central database, uploading the status of each sample, entering information about each patient in the central database, coordinating the flow of process between all teams, designating -80 ° C storage location for each mother vial and its respective RNA, and finally analyzing the RT-PCR results and uploading it in the institute, state and central database. This was in addition to generating hospital reports. The major limiting factor in the initial days was the fact that we had to retrieve and update the databases manually. But we soon recognized the issue and created bots that would automatically update the databases and retrieve the information. This is when the process became really quick and it took just about 24 hours to give the reports from the time of receiving the samples.
Overall, it was a grim time. Nevertheless, there is a lot of science that happened in this period all over the world. I never imagined in my lifetime that vaccine and drug development can move so quickly. But it did. I received my second dose of vaccine in march 2021. While fear from the lurking possibility of all emerging strains of concern is there but with vaccines, we can still breathe in peace.
Anyhow this is my story from last year. I would be happy to take questions. If you want to understand anything related to covid19 research, how drugs or vaccines work, or anything about how testing works please leave your questions in the comments below. Moreover, if you want me to cover any specific topic in this field, I would be glad to do so.
Signing out@scienceblocks.

References
- First confirmed case of COVID-19 infection in India: A case report
- PM Narendra Modi announces a national lockdown for 21 days starting midnight of 24-25 March
- Generation of SARS-CoV-2 Spike Pseudotyped Virus for Viral Entry and Neutralization Assays: A 1-Week Protocol
- hACE2 transgenic mouse model for CORONAVIRUS (COVID-19) research.
- A guide to laboratory diagnosis of Corona Virus Disease-19 for the gastroenterologists
- Multiplex Real-Time PCR
- Novel Coronavirus (SARS-CoV-2) Real-Time Multiplex RT-PCR Kit.

