Introduction: why I consider the following invitation to participate in "COVALIA" (title of new Clinical Study for a frontrunner in new wave of #vaccine #DNA #Gene #Therapy that aims to study the safety and immunogencitiy against COVID-19) as deserving widespread scrutiny.
Covigen: A new Covid-19 Vaccine Candidate in Australia
Boarding Direct Flight to Australia from Bangkok
2020 & 2021 so far - I am dubious of vaccines or new pharma medtech to the extreme, regardless of what it is lauded as and regardless of which biopharma behemoth conceived it. So when I received an invitation via email to participate as a guinea pig for what is titled "COVALIA" Clinical Study, it captured my attention.
When I read through it all to completion, picked up my jaw from the floor & reattached my gyrating spun-out head back onto its neck, I commenced a little bit of preliminary research. Frankly, I know next to nothing of gene therapies as a whole type of therapeutic treatment, except it is largely untested for safety or efficacy. I believe that there are no human safety trials for the same bio-medtech gene therapies to be used as a inoculation or vaccine against viral infections. I believe that this DNA Vaccine, named COVIGEN, is using DNA recoding, editing and/or mutation to affect human DNA and genome composition in a way which is going to be horrendously disastrous to the COVIGEN Vaxx Stabbed.
I have copied the 24 page Participant information and consent form which was emailed to me last month in the post below in it's entirety, along with errors and omissions contained within. It's also uploaded here to doc.droid
There is a lot to read and most readers might not want to go through it all to search for what a prospective DNA VAXX Clinical Study involves and whether there are clues as to what a DNA VAXX can do.
Therefore as a preamble, i've listed below what I find questionable and worthy of further investigation. I do not have any professional medical or scientific qualifications so what I list has moreso to do with questions of risk, liability, indemnity, use of language and I've interpreted as meaning something that's flat out clownworld crazy.
a1. "COVALIA" - etymological search.
Valiant, Valia (women's dresses),
Alia, Alius, Alias,
Coven, Covent, Covenant, Cove, Covo
a2. WTF is this shit and is it any way related?
- TechnoVALIA - related Australian-domiciled corporation
https://bionet-asia.com/our-partners/technovalia/ - The Principal Investigator is named as Associate Professor Peter Richmond on the title page of this document, yet if you refer to Technovalia's Media Release from March 2021 the Principal Investigator is named as being Associate Professor Nicholas Wood of University of Sydney Children’s Hospital Westmead Clinical School.
There could be basic explanation for this, maybe there's a separate Principal Investigator for each Clinical Study site location (being Adelaide, Perth and Sydney). - If one was to participate, how would one formulate the correct questions that relate to their personal health circumstances so they can understand or receive more information from the COVALIA Medtech Minions?
- How would one provide CONSENT to participate and what are the implicit assumptions regarding the onus to ensure that any CONSENT provided is unambiguously & of the strictest adherence to exemplifying INFORMED CONSENT?
- Who is Bionet Asia (Biotech firm based in Thailand which is creator of COVIGEN)?
- What is meant by:
"As you are aware, this virus caused an outbreak of an illness called CORONAVIRUS DISEASE-19 ("Covid-19") around the world."
pg 1 of 24. - All Guinea Pigs, Please note:
"In This Study It Will Be The First Time That This Particular DNA Vaccine is Given to Humans." - All Guinea Pigs, please note:
"It is an investigational Vaccine, which means it's not approved (licensed) by the Therapeutic Good Administration (TGA) or any other Regulatory Agency responsibile for approving drugs & vaccines anywhere else in the world" - Can't mix and match vaxxes if you participate in this trial. Fair enough but what is the significance of the 57 day delay period?
- Pharmajet (company) makes the needle-free injection devices which will be used on you. "Tropis" does intradermal delivery and "Stratis" does intramuscular delivery. Both devices not approved (licensed) by TGA.
- Both of these fluid stream needle-free injection devices are "pre-qualified" by your favourite Global Homogeneity Terror Squad, The W.H.O. (World Hell Order)
- The law requires (sometimes) the retention of your personal information even when you withdraw your consent in participating in the clinical trial.
- The trial must include all the data actually collected. Oh, really?
- "Randomised: means that you will receive by chance (like flipping a coin) the COVIGEN vaccine or placebo."
- 80% chance participant receives the COVIGEN Vaxx or placebo solution. 80% chance of receiving the study vaccine.
- It would be enlightening to receive a sample of the "Post-Dose Diary Card." pg 4 of 24
- Screening is fairly extensive including full medical, surgical and family history, nose swab test, piss test etc. Receiving any blood/plasma products or immunoglobulin within 90 days before starting clinical study trials is an automatic exclusion.
- Your GP (Doctor) will be informed of the study and they may provide your medical history to the study doctor, as they see relevant. Alarming, can you see the decision making on medical private information disclosure and decision making in your own best interest being deemed as not for you to decide?
- "Sentinel Groups - Sentinel Cohorts" pg 6 of 24
- Lots of double vaxx stabbings throughout the clinical study trial - "one in each arm."
- https://www.nhmrc.gov.au/research-policy/ethics/human-research-ethics-committees
- https://www.nhmrc.gov.au/about-us/freedom-information
- All your personal information is retaiend for up to 15 years for access to the Telethon Kids Institute, Sponsor and other affiliates. The information is "de-identified" to you and identifiable only as a code number
- "Currently there are no safety data about receiving one active vaccine made by one pharmaceuticalcompany followed by another active vaccine made by a different pharmaceutical company. You will be counselled regarding potential safety implications of receiving an registered vaccine after receiving the study vaccine. Hence, it is important that we continue to monitor you for safety until the end of the study." pg 13 of 24
- READ SECTION 14 starting on page 14 and going to page 17.
What Risks Do I Run By Taking Part? - "The DNA Vaccine cannot cause COVID-19 disease." pg 15 of 24
- "A trial of COVID-19 DNA vaccine was recently published by Innovio"
- Male participants:
If you are male, you should not plan to conceive a baby or donate sperm while participating in this study until at least 90 days following the last dose of study vaccine. The effect the vaccine has on your fertility is not known.
🖕You must wear a condom for all sexual intercourse as the study vaccine may affect your sperm risking the potential for an abnormal child being born.🖕
- Further research: https://www.alrc.gov.au/inquiry/protection-of-human-genetic-information/
- Further research, confirm if COVALIA is required to be declared here and whether it has been. http://www.ogtr.gov.au/

S T A R T OF 28 P A G E F O R M
Participant information and consent form
COVALIA: Phase I, double-blind, dose-ranging, randomised,
placebo-controlled trial to study the safety and
immunogenicity of a DNA-based vaccine against COVID-19
(COVIGEN) in healthy participants aged 18 to 75 years old
Sponsor Protocol Number: COV101
Location: 15 Hospital Avenue, Nedlands WA 6009
- Would you like to take part in this clinical study?
We would like to invite you to take part in our clinical study (also known as a clinical trial). This is because you are a healthy male or female aged between 18 and 75 years (inclusive) and may therefore be eligible for this trial.
This document tells you about the study and describes what will happen if you take part. If there is anything you don’t understand or want to know more about, please ask us.
You might also want to talk to a relative, a friend or your General Practitioner (GP) before you make up your mind.
If you decide you want to take part in the research project, you will be asked to sign the consent section. By signing it you are telling us that you:
Understand what you have read
Consent to take part in the research project
Consent to have the tests and treatments that are described
Consent to the use of your personal and health information as described.
You will be given a copy of this Participant Information and Consent Form to keep
- Why are we doing this research?
In this trial, we are evaluating the safety and tolerability of a new investigational DNA vaccine to protect against SARS CoV-2 virus, called COVIGEN, that is developed by a company called Bionet Asia. As you are aware, this virus has caused an outbreak of an illness called coronavirus disease19 (“COVID-19”) around the world.
In this study it will be the first time that this particular DNA vaccine is given to humans.
This is a phase 1 trial, meaning a small number of healthy adults are given different dose levels
to work out the best dose and whether it is tolerated and safe. It is an investigational vaccine,
which means it is not yet approved (licensed) by the Therapeutic Good Administration (TGA) in
Australia or any other Regulatory Agency responsible for approving drugs and vaccines
anywhere else in the world. . We will also measure immune responses to the vaccine through the
analysis of blood and saliva samples.
During the course of this trial other COVID-19 vaccines are likely to be approved for use in
Australia. Licensed vaccines have been rolled out in a targeted phase starting with high
risk groups first in February 2021.
If you do decide to take part in this trial then you will need to delay receiving an approved
COVID-19 vaccine for a period of at least 57 days after receiving the first dose of the study
vaccine.
A special device/s will be used to inject the vaccine that does not require the use of a needle
(needle-free injection) made by a company called Pharmajet. For delivery into the skin
(intradermally) a device called “Tropis” will be used, and for delivery into the muscle
(intramuscularly) a device called “Stratis”. These devices not yet approved (licensed) by the
Therapeutic Good Administration (TGA) in Australia for the administration of vaccines in
Australia, however Tropis is licensed for use to delliver influenza vaccine in the USA.
To deliver the vaccine into the muscle or skin, Stratis and Tropis Needle-Free Injectors are using
a narrow, precise fluid stream that goes through the skin, without a needle, in about 1/10 th of a
second. Like vaccines given by a needle, you will feel a sensation of pain when the fluid stream
goes through the skin, but this only lasts a short time.
Both devices have been approved (pre-qualified) by the World Health Organization for vaccinating
humans. Stratis is also licensed and approved for use in the USA to administer the flu vaccine.
Tropis has been safely used in other vaccine trials such as the polio vaccine.

- Do I have to take part?
Taking part in this study is voluntary (your choice). If you don’t wish to take part in the clinical study, you don’t have to. If you decide to take part and later change your mind, you can withdraw at any stage.
If you do withdraw your consent during the clinical trial, the research team will stop collecting
personal information from you. But they will keep the personal information they have collected up to that point. There is a good reason for this. Sometimes, the law requires it. It is also retained for accurate measurement. The trial results must include all the data actually collected.
Just to be clear on this point. We must keep any information about you we collect, up to the time you withdraw. The institution conducting the trial and the sponsor, University of Sydney (called the study “Sponsor”), has access to this information so they can check it is correct. If you do not agree with this then we cannot allow you to join the clinical trial.
- What is involved in the study?
We first need to confirm that the study is suitable for you.
For this trial we are looking to enroll male and female participants, aged from 18 to 75 (inclusive) who are in good health and who do not have a history of alcohol abuse or drug addiction.
To be eligible, prospective participants need to be able to understand the information in this document written in the English Language and not require the use of an interpreter. This trial is being conducted at 3 different locations in Australia, Scientia Clinical Research in Sydney, Telethon Kids Institute in Perth, and Women’s and Children’s Hospital in Adelaide. A total of 150 participants will be enrolled in 3 different groups, as explained below.
This is a randomised, double- blinded, placebo controlled trial.
- Randomised: means that you will receive by chance (like flipping a coin) the COVIGEN vaccine or placebo.
- Double-blinded: means that neither you, nor the study doctor or nurse, will know if you received the study vaccine or placebo until after the study has been completed.
- Placebo is a substance that looks like the study vaccine but does not contain any active ingredients in it; the placebo in this study contains only water and sodium chloride (salt).
- Controlled: means the study has been designed to compare the SARS-CoV-2 study vaccine to the placebo.
You will be assigned to 1 of 3 vaccination groups on Day 1 (the day on which you receive the first vaccination).
The three vaccination groups are:
- Intradermal
- Low dose intramuscular
- High dose intramuscular
A computer program will randomly decide which vaccination group you will go into. Then within each group, a computer program will randomly decide whether you will receive the COVIGEN vaccine or placebo solution. You have an 80% chance of receiving the study vaccine. Your study doctor will NOT be able to tell you which study group you have been assigned to or whether you will be receiving COVIGEN or placebo. Each participant will be given 2 injections, either with two active vaccines, or with two placebo vaccines on day 1 and day 29. This is outlined below.
Participants in all 3 groups will follow the same study procedures.
Neither you nor the study team will know which dose level of SARS-CoV-2 vaccine (3 possibilities)
you receive, or if you receive the placebo. This is done to make sure the results of the study cannot
be unfairly influenced by anyone. However, the study doctor can find out in the unlikely case of an
emergency.
The vaccination may be delayed to a later date if you are feeling unwell, have a fever or have high
blood pressure on the day of the planned vaccinations. You have to be well for at least 3 days prior
to the vaccination visit. As COVID-19 studies rapidly enrol participants over a short period of time
(1-2 weeks), it is possible that a delay in your vaccination may result in you not being able to take
part in the study. Also, it is possible that the targeted number of 150 participants is reached just as
you are about to start the study. The study doctor can then cancel your participation.
After your first vaccination, you will visit the study clinic 6 or 7 more times over a period of 12
months. The number of visits includes the second vaccination visit on Day 29. Each visit will last
about 1 to 2 hours. The main reason for these visits is to check you for any health changes or
problems, and at some visits to collect blood and saliva samples.
If you become ill with symptoms that could be COVID-19 infection, you should follow local
guidelines for potential positive COVID-19 cases. This would usually include having a COVID-19
test and self-isolating until the results are known. You should follow the local public health rules in
the area you live.
You will be asked to complete a paper diary Post Dose card from Day 1 (Day of vaccination) for 7
days following each of the vaccination days. The diary card has a list of symptoms which may
occur following vaccination. It will take approximately 5-10 minutes each day to complete the Post
Dose Diary card.
You will use a digital thermometer (which we will give to you) to measure your oral temperature
(under your tongue). You will then enter the temperature reading into your dairy. A measuring
ruler device (which we will give to you) will be used to measure the size of any redness or
swelling on your arm where your injection was given. You will then enter the measurement into
your diary. You will complete the home assessments for 7 days starting the day that you were
given your injection and then return the diary at the next visit.
You will also be asked to inform your study doctor of any medications, vaccinations, doctor visits,
or additional illnesses that you may experience during the duration of the study.
Visit 1 - Screening
To determine whether you meet the health criteria to participate in this study you will need to attend
one of the study clinics for a screening visit up to 30 days before your anticipated enrolment in the
study. This screening visit will take approximately 2 hours. Before the start of any procedures
associated with this study, including the screening to see that you meet the criteria, you will need
to sign an Informed Consent Form that states that you agree to participate voluntarily in this study
and to the study restrictions and the study procedures themselves. Avoid strenuous exercise and
alcohol consumption for approximately 72 hours prior to your screening appointment.
If you consent to the study procedures, the following assessments will be conducted:
- A full medical, surgical and family history including demographics will be collected.
- Vital signs (temperature, heart rate and blood pressure), height and weight will be
measured. - A sample of blood (approximately 15 mL, comparable to 3 teaspoons) will be collected
to perform routine laboratory tests e.g. biochemistry, haematology and screen for
specific infections including Hepatitis B and C, Human Immunodeficiency Virus (HIV),
and SARS-CoV-2, the virus that causes COVID-19.
Note: Just like other bloods results in this study, the results of your screening tests for
the infections will be provided to the Sponsor. These blood test results must be negative
for you to take part in the study. You will also receive information and counselling before
the test. Signing the consent form means that you agree to have this testing; it will not
be done without your consent. If your test results are positive, the study doctors will be
required to notify a government health department. You will also be provided with follow-up
counselling and medical advice. - Nasopharyngeal swab to exclude that you are positive for COVID-19.
- Urine sample will be collected to check for glucose (to exclude diabetes), protein and
blood (which can indicate kidney disease). If female, you will also have a urine
pregnancy test completed. - Please inform staff of any medications (including vitamins and supplements) that you
have taken, as some medications may interact with the study drug. - A review of any other treatments (e.g. blood products) and previous vaccinations.
The above tests, medical history and physical examination will determine whether you are
suitable for participation in this study. You will be given as much time as required to ensure
that you fully understand what is involved in the participation of this study. If you do consent to be involved in this study, you must be willing to cooperate with the directions of the study. Your study doctor and/or staff will review these with you. It is in your best interest to answer all questions completely and honestly.
Your history of any disease or disorder affecting any body system must be reported to the study
doctor, and they will determine whether this excludes you from participating in the study. This
includes the following:
- You must not have received, or be planning to receive, any vaccine other than the study
intervention within 30 days before and after each study vaccination - You must not be working in occupations with high risk of exposure to SARS-CoV-2 (e.g.
healthcare worker in direct care of COVID-19 patients. - You must not have received blood/plasma products or immunoglobulin, within 90 days before study intervention administration or plan to receive these during the study
Your General Practitioner will be informed of your participation in the study and they may provide details of your medical history to the study doctor, as they see relevant.
Study Treatment
If you are enrolled in this study, you will be assigned by chance into 1 of 3 groups consisting of 50 participants in each group. Each group will receive a different dose level of the COVIGEN vaccine, or placebo, administered either intradermal (into the skin) or intramuscular (into the muscle) using a device that does not require the use of a needle. Each participant will receive two injections, one
in each upper arm (as shown in the table below), at two different occasions: two injections on Day
1 and two injections on Day 29 of the study.
The following numbers of participants are included in the different groups:

Study groups
Each group in this study requires “sentinel groups”. You may be assigned to a sentinel group at
the Scientia Clinical Research site in Sydney ONLY. The Telethon Kids Institute will NOT be
involed in the sentinel cohorts for this study. Sentinel participants receive the study vaccine (or
placebo) before the rest of the participants to enable the researcher to assess any adverse effects
before a larger number of participants receive the study vaccine. This means that 5 participants
will be vaccinated before the other participants in each cohort. In each group, four of the sentinel
participants will receive COVIGEN, and one sentinel participant will receive placebo. Other
participants will only be enrolled into the group after it has been demonstrated that there are no
safety concerns in the sentinel participants within the first 2 days after vaccination.
Groups will be divided into 3 phases: screening phase (lasting up to 4 weeks), vaccination phase
(Day 1 and Day 29), and follow-up phase (between Day 29 to Day 365). The total duration of your
participation in the study is approximately 12 months.
The table below shows the procedures that you will complete at each point in the study.

Visit 2 = First Vaccination Visit (Day 1) (2-4 hours)
If the screening tests show that you can take part in the study, the following tests will be done at this
visit before you receive first injection of the study vaccine:
- Review of your health and any medications you have been taking, including any vaccinations,
and surgical procedures, or any new doctor or hospital visits. These questions will be asked at
each visit. - A physical examination will be performed.
- Vital signs: your blood pressure, heart rate and body temperature will be measured.
- Review of the entry conditions to check if this study is still right for you.
- A venous blood sample (up to 50 mL, comparable to 2-3 tablespoons) will be taken to assess
your body’s immune response (‘systemic immune responses’) before the study vaccine is
given. - A saliva sample to measure your immune response (‘mucosal immune responses’).
- Urine pregnancy test for females only.
- You will be randomly allocated to 1 of 3 treatment groups as outlined above. This assignment
will be done at Visit 2 only.
After completion of these tests, the study staff will give you the first injection of study vaccine.
You will be given 2 injections during this study visit: one in each upper arm.
After you receive the vaccinations, you will be observed for at least 60 minutes for any immediate major reactions to the study vaccine.
The study team will also provide you with instructions on what you should do after you leave the study clinic and when you should return to the study site.
Before you leave the study site on the day of your study injection, the study staff will show you how to answer the Post Dose diary card questions and use the digital thermometer and the measuring ruler device.
Visit 3 (Day 8) (30-60 minutes)
Review of your health and check to see whether you have any side effects or have had any new doctor or hospital visits.
A physical examination will be performed.
Your blood pressure, heart rate and body temperature will be measured. A blood sample (10mL, comparable to 2 teaspoons) for laboratory analysis of blood health markers (haematology, biochemistry,)
You will be given an interval diary card to use for the next 3 weeks.
Visit 4 (Day 15) (approximately 15 minutes)
- A phone call to check whether you have any side effects or have had any new doctor or hospital visits.
- If required a visit to the clinic can be arranged.
Visit 5 =Second Vaccination Visit (Day 29) (approximately 2 hours)
4 weeks after the first vaccinations (i.e. on Day 29), you will receive a second round of
vaccinations: one in each arm.You will be asked again about any medications you have been taking, including any
vaccinations.Review of your health and a check to see whether you have any side effects or have had any
new doctor or hospital visits.Review of your medical conditions to check if this study is still right for you.
Physical examination and vital sign measurements.
A urine sample for a urine pregnancy test for females only.
Blood samples (up to 50 mL, comparable to 2-3 tablespoons) will be taken:
- For laboratory analysis of blood health markers (haematology, biochemistry)
- Measure your body’s immune response (‘systemic immune responses’) to the first round of vaccinations
A saliva sample to measure your immune response (‘mucosal immune responses’) to the first round of vaccinations.
After you receive the vaccinations, you will be observed for at least 60 minutes for any immediate major reactions to the study vaccine.
You should record any reactions to the study vaccine in the Post Dose Diary Card for 7 days, starting on the evening of this visit.
Visit 6 (Day 36) (30-60 minutes)
- Review of your health and a check to see whether you have any side effects or have had any new doctor or hospital visits.
- Physical Examination: the study doctor will perform a health assessment.
- Your blood pressure, heart rate and body temperature will be measured.
- A blood sample (20 mL, comparable to 1 tablespoon) for laboratory analysis of blood health markers (haematology, biochemistry) and immune responses.
- A saliva sample to measure your immune response (‘mucosal immune responses’) to the vaccinations
- You will be given an interval diary card to use for the next 3 weeks
Visit 7 (Day 43) (approximately 15 minutes)
A phone call to check whether you have any side effects or have had any new doctor or hospital visits.
If required a visit to the clinic can be arranged.
Visit 8 (Day 57) and Visit 9 (Day 169) (30-60 minutes)
- Review of your health and a check to see whether you have any side effects or have had any
new doctor or hospital visits. - Physical Examination: the study doctor will perform a health assessment.
- Your blood pressure, heart rate and body temperature will be measured.
(No Visit 9???)
- Blood samples (up to 50 mL, comparable to 2-3 tablespoons) will be taken to assess your
immune responses (systemic immune responses) - A saliva sample to measure your immune response will be taken to assess your immune
response (‘mucosal immune responses)’ - We will collect and review your interval diary cards
Visit 10 (Day 365) = End of study visit (30-60 minutes)
- Review of your health and a check to see whether you have any side effects or have had any
new doctor or hospital visits. - Physical Examination: the study doctor will perform a health assessment.
- Your blood pressure, heart rate and body temperature will be measured.
- Blood samples (up to 50 mL, comparable to 2-3 tablespoons) will be taken to measure your body’s immune response (systemic immune responses) to the study vaccine
- A saliva sample to measure your immune response (‘mucosal immune responses’) to the vaccinations
What happens if I develop symptoms that could be COVID-19?
If you become unwell at any time during the study and have symptoms that may be COVID- 19 illness then you need to follow your local guidelines about testing and isolation. Your study site staff can help alert you to local options as needed. You will need to inform the study site staff that you have had a COVID-19 test and the result. We will ask you at each visit if you have been unwell or had a COVID19 test and the results.
Early Termination visit
If you withdraw from the study early or your Study Doctor withdraws you early from the study, where
possible, you will be asked to complete early termination procedures for safety reasons as listed in
the Schedule of Procedures above. These procedures will take approximately one hour to complete.
Blood Sampling
Over the course of the study blood samples will be taken 8 times.
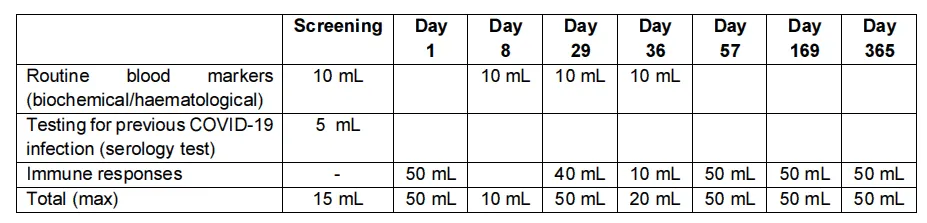
The total volume of blood taken for the entire 12-month study duration will be no more than 295 mL.
A standard blood donation is 470 mL in any 12-week period. You are advised not to donate any additional blood for 12 weeks after completing the study. As with all studies requiring blood donations, adequate rest and good eating habits are also advisable.
Blood will be collected for the following reasons:
- To check routine biochemical and haemotological markers.
- To assess antibody and cellular immune reponses to SARS-CoV-2 and related viruses.
- Extra blood may need to be taken during the study should repeat laboratory tests or
further analyses be needed. - If you are a woman who has stopped getting menstrual periods, then a blood test may
be done to confirm that post menopausal status. Your local investigator will discuss
with you whether this test needs to be performed - To study how some types of your white blood cells (cells of the immune system)
respond following vaccination.
Results of the routine laboratory tests will be reviewed by your study doctor. If any results are concerning, you will be notified and have further testing if needed.
5 Who is conducting and paying for this research?
This research project is being conducted by the Telethon Kids Institute and the sponsor is Sydney University. By participating in the study, there will be no costs payable by you.
6 Who has reviewed the study?
Child and Adolescent Health Service (CAHS) Human Research Ethics Committee (HREC) has reviewed this study in accordance with the National Statement on Ethical Conduct in Human Research (2007) and all relevant updates. This Statement has been developed to protect the interests of people who agree to participate in human research studies.
7 What if something new comes up during the study?
Sometimes during the course of a research project, new information becomes available about the treatment or vaccine being studied. If this happens, the study doctor will discuss with you what it means and whether you want to continue in the study. If you decide to continue in the clinical study, we will ask you to sign an updated consent form.
Also, on receiving new information, your study doctors might consider it to be in your best interest to withdraw you from the research project. If this happens, he/she will explain the reasons and arrange for your health care to continue.
8 What will happen to the confidential information about me?
We will keep all personal information confidential and securely stored. All of the collected data will be coded. No personal information about you, such as your name and address will leave the clinic, and in all study information sent out from the clinic you will be identified with a code number only.
9 What information will be collected, and how will it be stored?
All of your coded collected information will be kept by the Telethon Kids Institute and the Sponsor, University of Sydney and its affiliates for at least 15 years after the end of the study.
After the 15 years your identifying information at the Telethon Kids Institute will be permanently
destroyed.
Australian and New South Wales privacy law gives you the right to request access to your information that the researchers have collected and stored. The law also gives you the right to request corrections to any information about you that you disagree with. Please contact the study team (see page 19 of this document) if you would like to access your information.
Authorised representatives of University of Sydney and its affiliates, Southern Star Research, the
contract research organisaiton supporting this study, Human Research Ethics Committee, auditors
and pharmaceutical regulatory bodies (both Australian and foreign) may require access to your study
records to verify study procedures and/or data, including laboratories, pharmacies and contract
research organizations engaged by or on behalf of the Sponsor. Some of your information may be sent to people in other countries for these purposes. In all cases when dealing with your information, these people are required to comply with the privacy laws that protect you.
We will not disclose your information without your permission (including as permitted under this
informed consent form), except in compliance with the law. Information about you may be obtained
from your health records held at this institution and may be obtained from other health services for the purposes of research. Should you wish to cease treatment we would like the option to maintain follow up. If you sign the consent form, you agree to the study team accessing health records if they are relevant to your participation in this study.
Participants should also note that, the data collected from your participation in this study may be sent
overseas; the regulatory regimes governing data access and use in other countries may not be the
same as those that are in place in Australia and may not protect your information to the same extent
as the laws of Australia. Coded collected data will be transferred to the University of Sydney, Sydney,
Australia and its affiliates at the completion of the study. If you have any questions about this direct
them to your study doctor. All of your information will be de-identified.
10 What are my responsibilities during the study?
If you agree to participate in this study, you agree to be responsible for agreeing to follow the study
procedures according to our instructions. You also agree to comply with the other conditions in this
document. If you cannot, or do not wish to accept this responsibility, then you cannot participate in
the study.
11 Can I have other medicines during this clinical study?
You must tell us about any procedures or medicines you may be using. This is in your interest as well
as important for the study, because they may interact or interfere with the vaccine. You must tell us
about any prescription or over-the-counter medications, vitamins or herbal remedies you are taking.
You must tell us if you are having other alternative procedures (for example, osteopathy, chiropractic,
dietetics, acupuncture). You must also tell us about any changes to these while you are participating
in the clinical study.
12 Can I have other COVID-19 vaccines during this clinical study?
There are other COVID-19 vaccines registered for use in Australia and available to prevent SARS-
CoV-2 infection. There are also many other vaccines under development, and some vaccines will start to be used in mass vaccination programs in Australia during the period you are in the study.
If you wish to take part in this study you will need to delay receiving an approved COVID-19 vaccine
as part of Australia’s mass vaccination program until at least 57 days after receiving the first dose of
the study vaccination.
When you become eligible and are recommended to receive COVID-19 vaccines authorised for use
in Australia, then the study doctor can advise you on the options available for you.
What should I do if I want to get an registered COVID-19 vaccine?
In Australia the COVID-19 vaccine program is being rolled out in phases, starting with high risk groups first. You may need to wait several months before getting an registered vaccine especially if you are not in a high risk group. If you enrol on the trial and then later decide you wish to receive one of the registered vaccines available in Australia, we want you to let us know that you inted to get a registered COVID 19 vaccine. The study team can discuss the options available for you.
As this is a phase 1 trial, we don’t know whether the study vaccine works or not. In this study, you
have an 80% chance of receiving the study vaccine and 20% chance of receiving the placebo.
Currently there are no safety data about receiving one active vaccine made by one pharmaceutical
company followed by another active vaccine made by a different pharmaceutical company. You will
be counselled regarding potential safety implications of receiving an registered vaccine after
receiving the study vaccine. Hence, it is important that we continue to monitor you for safety until the
end of the study.
There are 2 options:
- You may continue in the study. You will be not receive a licenced vaccine until after the
end of study. - You can chose to have the registered vaccine and you will be told if you received the study
study vaccine or placebo (including the dose amount). We will still ask you to come for safety assessments in this case. We do not currently have any safety data receiving the study vaccine and then a licenced vaccine. As information on this becomes available we will tell you.
If you choose to get the registered vaccine, then this will need to be organised through your health care provider or the local state site. We will not be able to provide the vaccine through the study. Your health provider will need to know that you are participating in a coronavirus vaccine trial.
What should I do if I am waiting to receive an registered vaccine?
You should remain on the study and complete the study visits. You should stay on the study with
neither you nor the study staff knowing what you have received. This is the best way to continue to
get unbiased information. When you become eligible and wish to withdraw, please let the study staff
know and they will advise you of your options as outlined above.
Every person will be told whether they received active vaccine or placebo, either at the end of the
study or at the time they withdraw.
What will happen after I receive an registered vaccine?
If you decide to get a registered vaccine, then we would encourage you to continue safety assessments until the end of the study.
If you receive a registered vaccine and have a reaction/side effect – then you should do the following
- If a severe reaction – like a severe allergic reaction, call 000 or go to nearest hospital
- Call your healthcare provider who administered the vaccine
- Inform the study staff
13 What possible benefits might I get by taking part?
Taking part in this study may be of no direct benefit to you. However, the information generated during this study will guide us to make decisions on the further development of this COVID-19 vaccine in Australia.
In this study, some participants will receive the placebo and others will receive the experimental
vaccine. Even if you receive the experimental vaccine, its effectiveness is unknown, and you cannot
presume you will have protection against COVID-19 infection. You should continue with standard
precautions such as hand washing, masks if required, and social distancing.
14 What risks do I run by taking part?
One risk is that you will need to delay receiving another COVID-19 vaccine for at least 57 days after
receiving the first dose of the study vaccination.
Medical procedures, vaccinations, and tests may have side effects. You may experience no side
effects, some side effects or all of the side effects listed below. These side effects are most of the time mild, but can sometimes be moderate or severe. If you have any of these side effects, or are worried about them, talk with your study doctor. Your study doctor will also be looking out for side effects.
The study vaccine is an experimental vaccine and the main aim of this study is to measure the safety
and tolerability of the vaccine in healthy adults, and as a secondary aim to measure immune reponses.
Safety data from large Phase 3 trials withother COVID-19 vaccines made by genetic technology
including the Pfizer and Moderna mRNA vaccines show the most common side effects included
tiredness, headache, muscle aches, mild fever and some pain and redness at the injection site. These side effects were generally short-lived and participants made a complete recovery. There are at least 3 other DNA COVID-19 vaccines being tested in humans in other countries at the moment. Data on he side effects from one of these vaccines, made by Innovio, has recently been published as
discussed below..
The DNA vaccine cannot cause COVID-19 disease.
A previous small trial of a DNA vaccine for Zika virus, has been conducted, using the Stratis device in 15 participants. There were no serious side effects caused by the DNA Zika virus vaccine. The
main side effects from this vaccine given using the Stratis device, were local injection site reactions.
(80% of participants reported mild pain at the injection site). Aproximately half of the participants
reported any general reaction, mainly headache (33%) and tiredness/malaise (40%).
A trial of a COVID-19 DNA vaccine was recently published by Innovio. In this phase 1 trial safety
data was obtained from 39 participants who received 2 doses of a DNA vaccine (either 1mg and
2mg dose) given into the skin (intradermal). The vaccine was very well tolerated, with <15% of
participants experiencing any injection site reaction and one participant reporting nausea. There
were no serious reactions.
The known risks, side effects, and discomfort when people receive any vaccine include injection-site
reactions, which result in redness, itching, or a painful sensation at the place of the injection and
general reactions as explained below.
a) Local Reactions at vaccination site:
You may experience some discomfort at the injection site as the vaccination is given. This usually gets better within 5 minutes. Later, you might experience pain resulting in some difficulty moving your arm, but this should resolve within a few days. In addition to pain, you may experience redness, swelling, itchiness or warmth at the injection site. These reactions can occur even with a needle-free vaccine device, like Stratis or Tropis.
b) General reactions:
During the first 24-48 hours after vaccination you may experience flu-like symptoms such as muscle
aches, joint aches, feverishness, chills, headache, nausea, tiredness and/or feeling generally unwell.
These symptoms should usually resolve within a few days.
c) Serious Reactions:
As with any vaccine given by injection, very rarely people may have an allergic reaction. The allergic
reaction could be minor (rashes) or more severe including swelling of the face or lips (oedema),
wheezing (bronchospasm) or difficulty in breathing (dyspnoea). Other allergic reactions may include
hives or blisters.
If you have a history of severe allergic reactions then you will not be allowed to participate in
this study. If you have symptoms of an allergic reaction after vaccination you should seek
medical attention immediately. A severe allergic reaction (anaphylactic shock) may occur and
could be life threatening. If you think you are having an allergic reaction call 000 and/or go to
your local emergency department.
Reactions of the nervous system are extremely rare, but can cause an illness called Guillain-Barré
syndrome. This is a condition in which people can develop severe weakness and can be life
threatening. These adverse events have not previously been seen following administration of similar
vaccines.
A vaccine might make a disease worse if someone is exposed to the germ that causes the disease.
This is extremely rare. It has happened in some animal studies of vaccines for other coronaviruses
(the COVID-19 virus is a type of coronavirus). It has also happened in humans with a vaccine against
a respiratory virus called RSV. An independent safety committee (comprising of experts not involved
directly with the study) will monitor the safety aspect of the study. We will monitor for COVID-19
infection in the participants throughout the study. We will closely monitor what happens with their
illness. This expert group will compare the severity of illness between the study groups, as they can
see who has vaccine and placebo, if required. They will advise if the study needs to be halted or
stopped if there are concerns of safety. We will let you know if this happens.
Many side effects go away shortly after the intervention ends. However, sometimes side effects can
be serious, long lasting or permanent. Your study doctor will discuss the best way of managing any
side effects with you. Some unwanted effects may actually not be related to the study, nevertheless it
is important to document these.
Since there is always the possibility that some unexpected side effects may develop in some persons
who take this or any other drug, trained medical personnel are available at the Telethon Kids Institute
for immediate medical attention.
At regular intervals throughout the study, you will be questioned about how you are feeling. If you
experience any unusual signs or symptoms, you should report them to the study doctor as soon as
possible. The study doctor has the right to withdraw you from the study at any point for medical
reasons. With any new medicine or vaccine there is always a possibility of an unexpected side effect.
You will be provided with a 24h study contact number. If you experience unexpected events or
become in any way concerned you can use this to contact one of the study staff at any time We will
ask you to record these symptoms in the Diary cards as well.
There may be other risks that we do not know about yet. If we learn new information during the
study that might affect your decision to stay in the study, then we will tell you this new information.
Risks from Placebo: Some people taking part in this study will receive the placebo. The placebo in
this study is a saline solution (salt water) injection. Reactions that have been seen at the injection site
include pain, bruising, swelling and redness, which are known side effects for any injection.
What effect could the tests have on me?
Risks from Study Procedures:
Blood samples:
The risks and possible discomforts involved in taking blood include pain from inserting the needle, or
less often, swelling, bruising, or infection around the vein where the blood is collected. You may feel dizzy or may faint. If you have a previous history of feeling dizzy or fainting during blood sample
collection, you should talk to the study doctor.
Nasal Swabs:
The risks and possible discomforts involved in taking nasal swabs from you may include pain or
general discomfort. Sometimes it may cause minor bleeding from the nose.
Risks related to pregnancy
Female participants: If you are a woman and are able to have children, there is important information
for you to know about the pregnancy risk precautions for this study before you sign this form.
It is not known whether the study vaccine may affect an unborn child or nursing infant. For this reason, if you are breast-feeding, pregnant or plan to become pregnant during the study period, then you may not take part in this study.
If you are capable of becoming pregnant, you must use an acceptable method of birth control until at
least 90 days after the last study vaccine. Acceptable forms of birth control will be reviewed with you
at the beginning of the study and continued use will be monitored at scheduled visits.
You should inform the study staff immediately if you become pregnant during your participation in the
study. Your ongoing participation in the study will need to be discussed, including withdrawal. The
study doctor will discuss the risks of continuing with the pregnancy and the possible effects on the
foetus. Monitoring of your pregnancy will continue until the outcome is known. If required by country
regulations, you and your partner may be requested to sign a separate informed consent form prior to collection of data about the outcome of the pregnancy for scientific or security reasons.
Male participants: If you are male, you should not plan to conceive a baby or donate sperm while
participating in this study until at least 90 days following the last dose of study vaccine. The effect the
vaccine has on your fertility is not known. You must wear a condom for all sexual intercourse as the study vaccine may affect your sperm risking the potential for an abnormal child being born. In addition to a condom, your female partners must also use another form of contraception, such as an intrauterine device, diaphragm, oral contraceptives, injectable progesterone, subdermal implants or a tubal ligation.
You should inform your partner of your participation in the study and that contraception has been
strongly recommended. Further, you must agree that if your partner becomes pregnant while you are
on the study, you will advise the study doctor who will then provide you with an authorisation form to
present to your partner. If she is in agreement, that authorisation will function as consent to approve
the study doctor’s access to medical information to allow monitoring of the pregnancy, and the birth
and the health of the child up to one year of age.
continues in posted comment below
